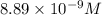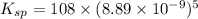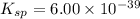Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Anurse practitioner prepares an injection of promethazine, an antihistamine used to treat allergic rhinitis. if the stock bottle is labeled 25 mg/ml and the order is a dose of 11.0 mg , how many milliliters will the nurse draw up in the syringe?
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
The molar solubility of ba3(po4)2 is 8.89 x 10-9 m in pure water. calculate the ksp for ba3(po4)2. t...
Questions




History, 25.02.2020 19:15

Mathematics, 25.02.2020 19:15

Health, 25.02.2020 19:16





Mathematics, 25.02.2020 19:16





Chemistry, 25.02.2020 19:17



Arts, 25.02.2020 19:17

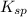 is
is 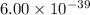
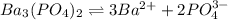
![K_{sp}=[Ba^{2+}]^3[PO_4^{3-}]^2](/tpl/images/0124/1385/f39cc.png)
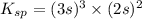
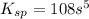
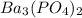 = s =
= s =