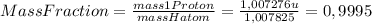Chemistry, 24.07.2019 13:00 briiannamb
What is the fraction of the hydrogen atom's mass (11h) that is in the nucleus? the mass of proton is 1.007276 u, and the mass of 11h atom is 1.007825 u. express your answer using four significant figures?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:40
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1


Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
What is the fraction of the hydrogen atom's mass (11h) that is in the nucleus? the mass of proton i...
Questions



Biology, 08.07.2019 06:30





Mathematics, 08.07.2019 06:30




Mathematics, 08.07.2019 06:30

History, 08.07.2019 06:30


Mathematics, 08.07.2019 06:30

History, 08.07.2019 06:30


Mathematics, 08.07.2019 06:30

Health, 08.07.2019 06:30

English, 08.07.2019 06:30