
Chemistry, 25.07.2019 03:00 ewymer3901
Some antacid tablets contain aluminum hydroxide. the aluminum hydroxide reacts with stomach acid according to the equation: ai(oh)3 +3hciaici, +3h2o. determine the moles of acid neutralized if a tablet contains 0.200 mol of al(oh)3

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 23.06.2019 02:20
Which of the following will cause an increase in the acceleration of an object? increase force decrease force increase mass decrease mass
Answers: 1

Chemistry, 23.06.2019 05:00
He nucleus contains the cells genetic material in the form of dna. dna is organized into our chromosomes, which are made up of thousands of that determine our traits.
Answers: 1

Chemistry, 23.06.2019 09:00
Which question could be best answered using the process of scientific inquiry? do different plates have different rock compositions? why did it take so long to develop the theory of plate tectonics? what are different cultural myths caused by plate tectonics? do plates move intentionally to cause volcanic eruptions?
Answers: 3
You know the right answer?
Some antacid tablets contain aluminum hydroxide. the aluminum hydroxide reacts with stomach acid acc...
Questions

Physics, 21.01.2021 01:00

Mathematics, 21.01.2021 01:00

Mathematics, 21.01.2021 01:00


Mathematics, 21.01.2021 01:00

Health, 21.01.2021 01:00

English, 21.01.2021 01:00


Mathematics, 21.01.2021 01:00

Mathematics, 21.01.2021 01:00

Mathematics, 21.01.2021 01:00


Mathematics, 21.01.2021 01:00

Mathematics, 21.01.2021 01:00

Chemistry, 21.01.2021 01:00



Mathematics, 21.01.2021 01:00

Mathematics, 21.01.2021 01:00

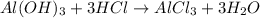
 of hydrochloric acid.
of hydrochloric acid.

