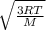Chemistry, 25.07.2019 07:00 robertojr24ov5pel
Calculate the root mean square (rms) average speed of the atoms in a sample of xenon gas at 0.15 atm and -55c

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
Calculate the root mean square (rms) average speed of the atoms in a sample of xenon gas at 0.15 atm...
Questions


Mathematics, 18.03.2021 01:20






Arts, 18.03.2021 01:20




Mathematics, 18.03.2021 01:20


Mathematics, 18.03.2021 01:20

Biology, 18.03.2021 01:20

Mathematics, 18.03.2021 01:20

Computers and Technology, 18.03.2021 01:20