
Chemistry, 25.07.2019 07:30 ramentome7542
When a 3.80 g sample of magnesium nitride (mw 101g/mol) is reacted with 3.30 g of water, 3.60 g of mgo is formed. what is the percent yield of this reaction? mg3n2 + 3 h2o --> 2 nh3 + 3 mgo

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle
Answers: 3

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1


Chemistry, 23.06.2019 06:10
How can liquids be seperated by density a the liquids are absorbed onto a paper b the liquids are turned into seperate vapors c the liquids are collected as they evaporate d the liquids are allowed to seperate into layers
Answers: 1
You know the right answer?
When a 3.80 g sample of magnesium nitride (mw 101g/mol) is reacted with 3.30 g of water, 3.60 g of m...
Questions



Mathematics, 08.12.2020 01:50

Mathematics, 08.12.2020 01:50


Computers and Technology, 08.12.2020 01:50


Chemistry, 08.12.2020 01:50

Mathematics, 08.12.2020 01:50


Mathematics, 08.12.2020 01:50

Mathematics, 08.12.2020 01:50

English, 08.12.2020 01:50

English, 08.12.2020 01:50

English, 08.12.2020 01:50

Geography, 08.12.2020 01:50

Mathematics, 08.12.2020 01:50


Mathematics, 08.12.2020 01:50

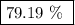 .
.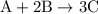
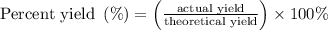 ......(1)
......(1)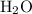 and is as follows:
and is as follows:
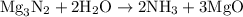 ……. (2)
……. (2)
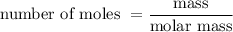
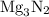 is
is 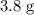 .
.
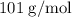 .
.
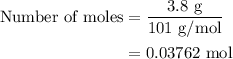
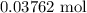 .
.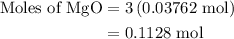
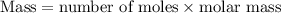
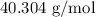 .
.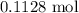 .
.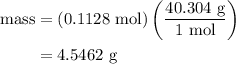
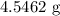 .
.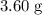 .
.
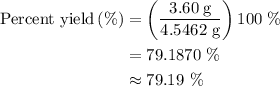
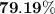 .
.


