
Two moles of propene (c3h6) gas react with nine moles of oxygen (o2) gas to produce six moles of carbon dioxide gas (co2) and six moles of water gas (h2o). using this description of the reaction, write the corresponding chemical equation, showing the appropriate coefficients for each reactant and product. (include states-of-matter under the given conditions in your answer.)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3


Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2
You know the right answer?
Two moles of propene (c3h6) gas react with nine moles of oxygen (o2) gas to produce six moles of car...
Questions

Arts, 10.06.2021 22:10



Spanish, 10.06.2021 22:10


Mathematics, 10.06.2021 22:10



Biology, 10.06.2021 22:10

Biology, 10.06.2021 22:10






Chemistry, 10.06.2021 22:10

Mathematics, 10.06.2021 22:10


Medicine, 10.06.2021 22:10

Arts, 10.06.2021 22:10

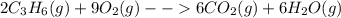
 to the gaseous state-of-matter at which the chemical reaction is carried out. Such coefficients are useful to respect the law of conservation of mass.
to the gaseous state-of-matter at which the chemical reaction is carried out. Such coefficients are useful to respect the law of conservation of mass.

