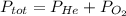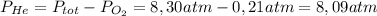He partial pressure of oxygen gas in our atmosphere is 0.21 atm. this is the partial pressure at which human lungs have evolved to be able to breathe this gas. a scuba diver, will thus still have to breath oxygen at this pressure even when diving way down in the water. if a mixture of helium and oxygen (heliox) in his tank is at a pressure of 8.30 atm, what must the partial pressure be of helium to keep the partial pressure of oxygen at 0.21 atm?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

You know the right answer?
He partial pressure of oxygen gas in our atmosphere is 0.21 atm. this is the partial pressure at whi...
Questions

Mathematics, 01.12.2020 22:40

Mathematics, 01.12.2020 22:40



Mathematics, 01.12.2020 22:40

Mathematics, 01.12.2020 22:40



Spanish, 01.12.2020 22:40

Mathematics, 01.12.2020 22:40

History, 01.12.2020 22:40


Mathematics, 01.12.2020 22:40


English, 01.12.2020 22:40

Arts, 01.12.2020 22:40

Mathematics, 01.12.2020 22:40

Biology, 01.12.2020 22:40

Mathematics, 01.12.2020 22:40

Mathematics, 01.12.2020 22:40