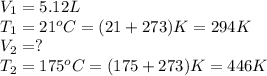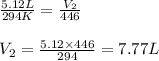Chemistry, 28.07.2019 07:00 bryson9604
Afixed quantity of gas at 21 °c exhibits a pressure of 752 torr and occupies a volume of 5.12 l. (a) calculate the volume the gas will occupy if the pressure is increased to 1.88 atm while the temperature is held constant. (b) calculate the volume the gas will occupy if the temperature is increased to 175 °c while the pressure is held constant.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1
You know the right answer?
Afixed quantity of gas at 21 °c exhibits a pressure of 752 torr and occupies a volume of 5.12 l. (a)...
Questions


Physics, 11.10.2019 11:20

Mathematics, 11.10.2019 11:20

Social Studies, 11.10.2019 11:20







Social Studies, 11.10.2019 11:30


Social Studies, 11.10.2019 11:30

Computers and Technology, 11.10.2019 11:30

Mathematics, 11.10.2019 11:30


History, 11.10.2019 11:30



Mathematics, 11.10.2019 11:30

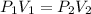
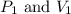 are initial pressure and volume.
are initial pressure and volume.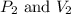 are final pressure and volume.
are final pressure and volume.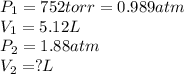
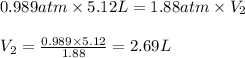
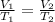
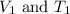 are the initial volume and temperature of the gas.
are the initial volume and temperature of the gas.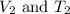 are the final volume and temperature of the gas.
are the final volume and temperature of the gas.