
Chemistry, 30.07.2019 02:00 ericgalo808
The rate of disappearance of hbr in the gas phase reaction 2hbr(g)→h2(g)+br2(g) is 0.140 m s-1 at 150°c. the rate of appearance of h2 is m s-1.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2
You know the right answer?
The rate of disappearance of hbr in the gas phase reaction 2hbr(g)→h2(g)+br2(g) is 0.140 m s-1 at 15...
Questions


History, 02.08.2019 01:30


Mathematics, 02.08.2019 01:30

Biology, 02.08.2019 01:30





Mathematics, 02.08.2019 01:30



Chemistry, 02.08.2019 01:30

Computers and Technology, 02.08.2019 01:30

Mathematics, 02.08.2019 01:30

History, 02.08.2019 01:30


History, 02.08.2019 01:30

Mathematics, 02.08.2019 01:30

English, 02.08.2019 01:30

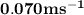
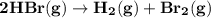
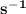
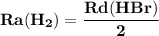
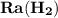 - Rate of appearance of Hydrogen
- Rate of appearance of Hydrogen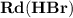 - Rate of disappearance of HBr
- Rate of disappearance of HBr 


