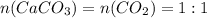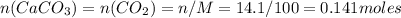Chemistry, 31.07.2019 22:00 CadenClough13
How many liters of carbon dioxide will be formed from the decomposition of 14.1g of calcium carbonate (at stp)?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1


Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2
You know the right answer?
How many liters of carbon dioxide will be formed from the decomposition of 14.1g of calcium carbonat...
Questions


Geography, 06.10.2019 12:00

Mathematics, 06.10.2019 12:00

Mathematics, 06.10.2019 12:00

History, 06.10.2019 12:00

Social Studies, 06.10.2019 12:00



English, 06.10.2019 12:00

Social Studies, 06.10.2019 12:00


History, 06.10.2019 12:00

English, 06.10.2019 12:00

Chemistry, 06.10.2019 12:00

Chemistry, 06.10.2019 12:00

History, 06.10.2019 12:00

Social Studies, 06.10.2019 12:00


Social Studies, 06.10.2019 12:00

English, 06.10.2019 12:00

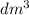 , ie the value of the molar volume (Vm) is 22.4
, ie the value of the molar volume (Vm) is 22.4 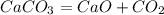
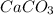 and
and 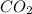 have the following amount of substance relationship:
have the following amount of substance relationship: