
Chemistry, 02.08.2019 00:30 seannalove4148
For real gases, how does a change in pressure affect the ratio of pv to nrt? a. the ratio decreases as pressure increases. b. the ratio increases as pressure increases. c. the ratio is constant at all pressures.

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2

Chemistry, 23.06.2019 07:00
Scuba divers use tanks of compressed air to them breathe. gases can be compressed because?
Answers: 1

Chemistry, 23.06.2019 08:00
Amechanical wave that transports a lot of energy will have a
Answers: 2

Chemistry, 23.06.2019 09:30
The allotropes of carbon include a variety of structures that include three-dimensional tetrahedral lattices, planes of hexagonal rings, cylindrical tubes of hexagonal rings, and spheres of five- and six-membered rings. similar shapes of network covalent atomic solids are possible with carbon nitride, boron, and pure silicon (e.g., silicene is a graphene-like allotrope of pure silicon). in contrast, silicates exist as either highly ordered or amorphous (more random) three-dimensional lattices. what could explain why there are there no naturally occurring sheets, stacked sheets, cylindrical tubes, or spheres of network covalent atomic solids composed of silicon and oxygen (sio2)? would pure silicate structures make good lubricants or good electrical conductors?
Answers: 3
You know the right answer?
For real gases, how does a change in pressure affect the ratio of pv to nrt? a. the ratio decreases...
Questions

Mathematics, 28.01.2020 17:43






Mathematics, 28.01.2020 17:43




Chemistry, 28.01.2020 17:43







Social Studies, 28.01.2020 17:43

History, 28.01.2020 17:43

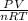 is either greater than or smaller than 1.
is either greater than or smaller than 1.

