
Chemistry, 02.08.2019 06:00 RickyGotFanz4867
At a certain temperature, this reaction establishes an equilibrium with the given equilibrium constant, kc. if, at this temperature, 2.20 mol of a and 3.70 mol of b are placed in a 1.00-l container, what are the concentrations of a, b, and c at equilibrium?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 23.06.2019 10:00
Which number should be placed before f2 on the reactants side equation to make equation balanced? xe + > xef4
Answers: 1
You know the right answer?
At a certain temperature, this reaction establishes an equilibrium with the given equilibrium consta...
Questions



Chemistry, 05.03.2020 12:01



English, 05.03.2020 12:01





History, 05.03.2020 12:02

Mathematics, 05.03.2020 12:02




Mathematics, 05.03.2020 12:03




![[C]_{eq}=4(0.733M)=2.932M](/tpl/images/0160/7486/db26c.png)
![[A]_{eq}=2.20M-3(0.733M)=0.001M](/tpl/images/0160/7486/5ae76.png)
![[B]_{eq}=3.70M-2(0.733M)=2.23M](/tpl/images/0160/7486/3f04f.png)
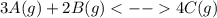
![[A]_0=\frac{2.20mol}{1.00L} =2.20M](/tpl/images/0160/7486/dc9d9.png)
![[B]_0=\frac{3.70mol}{1.00L} =3.70M](/tpl/images/0160/7486/d8b18.png)
![Kc=\frac{[C]_{eq}^4}{[A]_{eq}^3[B]_{eq}^2}](/tpl/images/0160/7486/3c943.png)
 , one obtains:
, one obtains: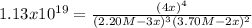

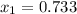 as the other one produces a negative concentration of A at equilibrium, therefore, the requested concentration turn out into:
as the other one produces a negative concentration of A at equilibrium, therefore, the requested concentration turn out into:

