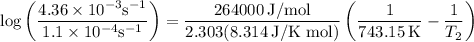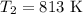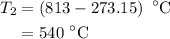Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2
You know the right answer?
The rate constant of a reaction is 1.1 × 10-4 s-1 at 470 °c, and the activation energy is 264 kj/mol...
Questions

Mathematics, 23.08.2021 14:00

Mathematics, 23.08.2021 14:00


English, 23.08.2021 14:00

Mathematics, 23.08.2021 14:00


Physics, 23.08.2021 14:00

History, 23.08.2021 14:00

Business, 23.08.2021 14:00



Mathematics, 23.08.2021 14:00

Biology, 23.08.2021 14:00

Arts, 23.08.2021 14:00

Mathematics, 23.08.2021 14:00

Social Studies, 23.08.2021 14:00

Mathematics, 23.08.2021 14:00

Mathematics, 23.08.2021 14:00


History, 23.08.2021 14:00

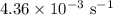 comes out to be
comes out to be  .
.
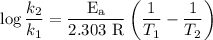 …… (1)
…… (1)
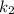 is rate constant at temperature
is rate constant at temperature 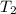 .
.
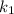 is rate constant temperature
is rate constant temperature 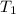 .
.
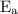 is activation energy.
is activation energy.
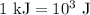
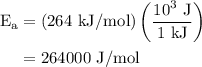
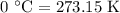
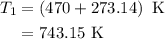
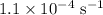 .
.