
Chemistry, 03.08.2019 05:30 krystlemiller4307
Acombustion analysis of 5.214 g of a compound yields 5.34 g co 2 , 1.09 g h 2 o, and 1.70 g n 2 . if the molar mass of the compound is 129.1 g/mol, what is the chemical formula of the compound?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aspirin has a density of 1.40 g/cm3 what is the volume in cubic centimeters of a tablet weighing 320 mg ?
Answers: 1

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1
You know the right answer?
Acombustion analysis of 5.214 g of a compound yields 5.34 g co 2 , 1.09 g h 2 o, and 1.70 g n 2 ....
Questions







Mathematics, 22.05.2020 02:04

Mathematics, 22.05.2020 02:04


Mathematics, 22.05.2020 02:04






Mathematics, 22.05.2020 02:04




Mathematics, 22.05.2020 02:04

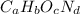 . The mass and molar mass of compound is 5.214 g and 129.1 g/mol respectively.
. The mass and molar mass of compound is 5.214 g and 129.1 g/mol respectively.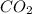 , 1.09 g of
, 1.09 g of 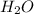 and 1.70 g of
and 1.70 g of 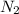 . First number of moles of carbon, hydrogen, oxygen and nitrogen to compare the molar ratio.
. First number of moles of carbon, hydrogen, oxygen and nitrogen to compare the molar ratio.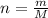
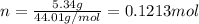
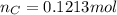
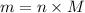
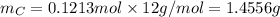
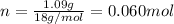
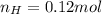
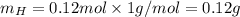
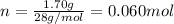
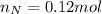
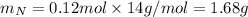
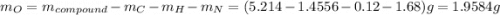
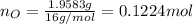
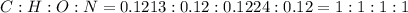
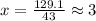
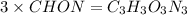
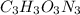 .
.

