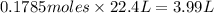Chemistry, 30.07.2019 13:40 herringalyssa
Water can be formed according to the equation: 2h2 (g) + o2 (g) → 2h2o (g) if 8.0 l of hydrogen is reacted at stp, exactly how many liters of oxygen at stp would be needed to allow complete reaction?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an oxidation-reduction reaction, oxidation is what happens when a reactant
Answers: 1

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1
You know the right answer?
Water can be formed according to the equation: 2h2 (g) + o2 (g) → 2h2o (g) if 8.0 l of hydrogen is...
Questions

English, 15.02.2021 14:00

Mathematics, 15.02.2021 14:00

Chemistry, 15.02.2021 14:00


Mathematics, 15.02.2021 14:00

Mathematics, 15.02.2021 14:00

Mathematics, 15.02.2021 14:00

Mathematics, 15.02.2021 14:00


Mathematics, 15.02.2021 14:00





Mathematics, 15.02.2021 14:00



Mathematics, 15.02.2021 14:00


Biology, 15.02.2021 14:00

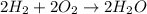
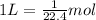
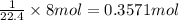
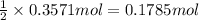 of oxygen
of oxygen