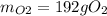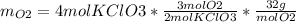The following equation is one way to prepare oxygen in a lab. 2kclo3 → 2kcl + 3o2 molar mass info: mm o2 = 32 g/mol mm kcl = 74.55 g/mol mm kclo3 = 122.55 g/mol if 4.00 moles of kclo3 are totally consumed, how many grams of oxygen gas would be produced? 192 g 6.00 g 85.3 g 735 g

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 23.06.2019 09:30
Organisms that live in the alpine and taiga biomes have developed unique adaptations that aid in their survival. moss campion is one of the plants found in the alpine biome. it has small leaves and a cushion shape that protect it from the wind and freezing temperatures in the alpine. how has the moss campion adapted to enable its survival in the alpine biome? a. waxy needles b. cone-shaped c. thin trunks d. low-growing
Answers: 1
You know the right answer?
The following equation is one way to prepare oxygen in a lab. 2kclo3 → 2kcl + 3o2 molar mass info:...
Questions

Chemistry, 21.09.2019 15:00


English, 21.09.2019 15:00

English, 21.09.2019 15:00

Social Studies, 21.09.2019 15:00

Physics, 21.09.2019 15:00

Mathematics, 21.09.2019 15:00


Mathematics, 21.09.2019 15:00

Mathematics, 21.09.2019 15:00

Computers and Technology, 21.09.2019 15:00

History, 21.09.2019 15:00


English, 21.09.2019 15:00


History, 21.09.2019 15:00

English, 21.09.2019 15:00

Chemistry, 21.09.2019 15:00