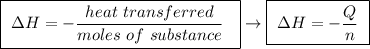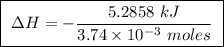Chemistry, 22.07.2019 18:00 ttwright24
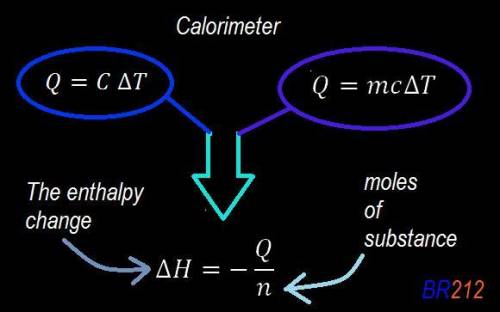
Abomb calorimeter has a heat capacity of 2.47 kj/k. when a 0.105-g sample of ethylene (c2h4) was burned in this calorimeter, the temperature increased by 2.14 k. calculate the energy of combustion for one mole of ethylene. a. –0.259 kj/mol b. –50.3 kj/mol c. –5.29 kj/mol d. –1.41 × 103 kj/mol e. –660 kj/mol

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2
You know the right answer?
Abomb calorimeter has a heat capacity of 2.47 kj/k. when a 0.105-g sample of ethylene (c2h4) was bur...
Questions





Mathematics, 23.07.2019 14:30


Mathematics, 23.07.2019 14:30


History, 23.07.2019 14:30


History, 23.07.2019 14:30

English, 23.07.2019 14:30

Health, 23.07.2019 14:30


Computers and Technology, 23.07.2019 14:30


Mathematics, 23.07.2019 14:30

Spanish, 23.07.2019 14:30

Mathematics, 23.07.2019 14:30

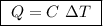
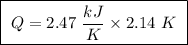
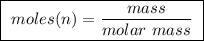
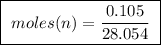
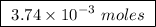 of ethylene.
of ethylene.