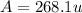Chemistry, 08.07.2019 23:10 KillerSteamcar
Anewly discovered element, y, has two naturally occurring isotopes. 90.3 percent of the sample is an isotope with a mass of 267.8 u, and 9.7 percent of the sample is an isotope with a mass of 270.9 u. what is the weighted average atomic mass for this element? 269.4 u 268.1 u 269.0 u 270.6 u

Answers: 1
Another question on Chemistry

Chemistry, 20.06.2019 18:04
If a certain battery supplies 1.0×108 electrons per second to the negative terminal and the battery contains 7.00 moles of electrolytic solution (which means the solution contains 7.00 moles of hso4), what fraction of the solution undergoes a chemical reaction each second
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2
You know the right answer?
Anewly discovered element, y, has two naturally occurring isotopes. 90.3 percent of the sample is an...
Questions






Mathematics, 14.05.2021 22:10



Arts, 14.05.2021 22:10





Mathematics, 14.05.2021 22:10


Biology, 14.05.2021 22:10


Mathematics, 14.05.2021 22:10

Social Studies, 14.05.2021 22:10

Spanish, 14.05.2021 22:10

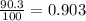
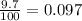
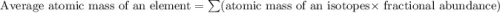
![A=\sum[267.8\times 0.903+270.9\times 0.097]](/tpl/images/0067/2857/bf750.png)