Carbon disulfide is produced by the reaction listed below:
--->
If you started the...


Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 09:00
Plz mark brainliest 30 points1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s.2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1


Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
You know the right answer?
Questions

Mathematics, 18.12.2019 06:31



Mathematics, 18.12.2019 06:31

History, 18.12.2019 06:31

Social Studies, 18.12.2019 06:31



Mathematics, 18.12.2019 06:31


Mathematics, 18.12.2019 06:31


Mathematics, 18.12.2019 06:31



History, 18.12.2019 06:31


Mathematics, 18.12.2019 06:31


Mathematics, 18.12.2019 06:31

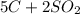 --->
--->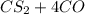
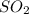 and excess carbon, what amount, in moles, of
and excess carbon, what amount, in moles, of 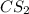 will be produced?
will be produced?

