
Chemistry, 01.02.2022 21:50 payshencec21
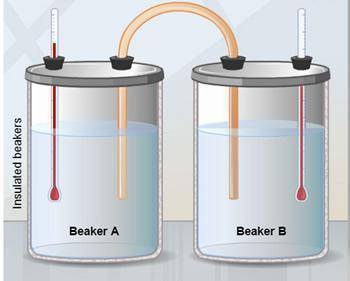
The two insulated beakers shown contain equal amounts of identical liquids. The temperature of Beaker A is 80°C. The temperature of Beaker B is 50°C. A copper rod connects the beakers. The system is then left alone for several hours. What would you expect to find when the system is examined after this time?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aaspirin has a density of 1.40 g/cm^3 what is the volume in cubic centimeters of a tablet weighing 320 mg?
Answers: 3

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3
You know the right answer?
The two insulated beakers shown contain equal amounts of identical liquids. The temperature of Beake...
Questions

Mathematics, 13.11.2019 01:31

Mathematics, 13.11.2019 01:31








Mathematics, 13.11.2019 01:31



Mathematics, 13.11.2019 01:31



Mathematics, 13.11.2019 01:31

History, 13.11.2019 01:31

Mathematics, 13.11.2019 01:31


Mathematics, 13.11.2019 01:31




