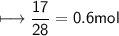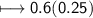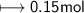Chemistry, 16.01.2022 05:50 ashleyvalles16
Calculate the number of moles of SO2 required to produce 17 g of CO in the following chemical equation: 5 C + 2 SO2 → CS2 + 4 CO

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:00
Use examples from the article to explain one positive and one negative effect that chemistry has had on society
Answers: 2

Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 23.06.2019 07:00
If you used the method of initial rates to obtain the order for no2, predict what reaction rates you would measure in the beginning of the reaction for initial concentrations of 0.200 m, 0.100 m, & 0.050 m no2.
Answers: 3

Chemistry, 23.06.2019 09:00
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180 g/mol) needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.
Answers: 1
You know the right answer?
Calculate the number of moles of SO2 required to produce 17 g of CO in the following chemical equati...
Questions





History, 10.03.2020 00:09