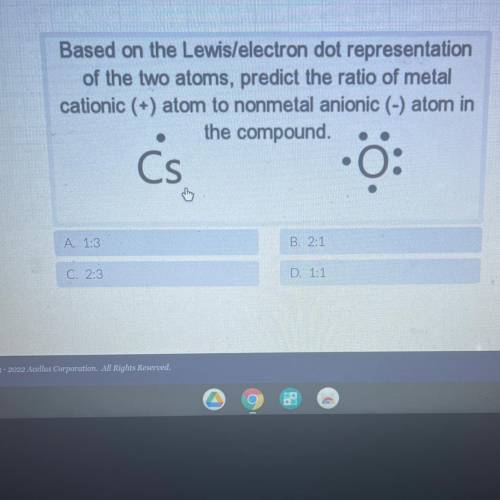
Based on the Lewis/electron dot representation
of the two atoms, predict the ratio of metal
...

Chemistry, 12.01.2022 01:00 Dreambig85
Based on the Lewis/electron dot representation
of the two atoms, predict the ratio of metal
cationic (+) atom to nonmetal anionic (-) atom in
the compound.
Cs
0:
A. 1:3
B. 2:1
C. 2:3
D. 1:1

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2



Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
You know the right answer?
Questions



Mathematics, 07.04.2020 20:36

Computers and Technology, 07.04.2020 20:36


Mathematics, 07.04.2020 20:36

Health, 07.04.2020 20:36

Mathematics, 07.04.2020 20:36


English, 07.04.2020 20:36



Biology, 07.04.2020 20:36


Mathematics, 07.04.2020 20:36


History, 07.04.2020 20:36

Biology, 07.04.2020 20:36


Mathematics, 07.04.2020 20:36



