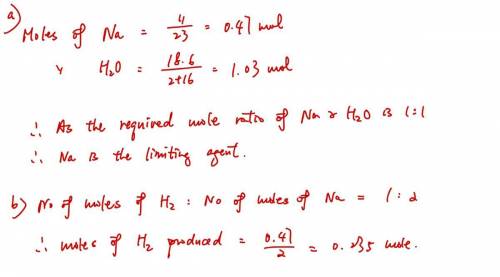A sample of 11.0 g of sodium is reacted with 18.6 g of water to produce sodium hydroxide and hydrogen gas. Using the balanced equation below, predict which is the limiting reactant and the maximum amount in moles of hydrogen gas that can be produced. 2Na+ 2H2O + 2NaOH + H2

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1

Chemistry, 23.06.2019 06:40
1.) which of the following is a molecule but not a compound? a.he b.f2 c.h2o d.ch4 2.) what is a physical combination of substances? a.a compound b.a molecule c.a mixture d.an element 3.) what is a chemical combination of substances? a.a compound b.an atom c.a mixture d.an element 4.) what is the relationship between the solute and solvent in a solution? a.they form a compound b.they form a mixture c.they form molecules d.they form chemical bonds 5.) the gases in air dissolve in water. what would be one way to reduce the amount of a gas dissolved in water? a.add more water b.reduce the air pressure c.increase the air pressure d.stir the water 6.) how would you determine the solubility of a substance? a.find how well it dissolved various substances. b.find the mass and the volume of the substance. c.find the temperature at which the substance evaporated. d.find how much i was able to dissolve in a solute. 7.) the periodic table organizes all of the kinds of a.molecules. b.compounds. c.atoms. d.ions. 8.)what distinguishes two substances combined to become a compound vs. two substances combined to become a mixture? a.whether they can be easily separated b.whether they chemically bond together c.whether they both are visible d.whether they are heterogeneous 9.) the principle components of air are: n2 78% o2 21% ar 0.95% co2 0.038% this is a solution of a.molecules and atoms. b.molecules. c.compounds and molecules. d.atoms.
Answers: 1
You know the right answer?
A sample of 11.0 g of sodium is reacted with 18.6 g of water to produce sodium hydroxide and hydroge...
Questions



English, 09.10.2021 07:10

Physics, 09.10.2021 07:10

Mathematics, 09.10.2021 07:10


Mathematics, 09.10.2021 07:10


SAT, 09.10.2021 07:10

Mathematics, 09.10.2021 07:10

History, 09.10.2021 07:10

Mathematics, 09.10.2021 07:10

Mathematics, 09.10.2021 07:10





Biology, 09.10.2021 07:10

Mathematics, 09.10.2021 07:10