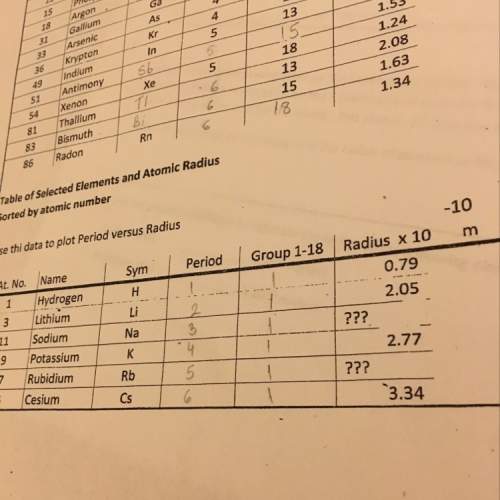
Please help I’m stuck
b. Water has a specific heat of 4.184 J/gºC. If the temperature of 250 g of
water changes from 22.9°C to 14.7°C, how much heat energy was removed
from the water?
C. A sample of aluminum (c = 0.900 J/gºC) is heated from 20.8°C to 38.7 °C
using 3,750 J of energy. What is the mass of the aluminum sample?
d. Determine the final temperature of sample with a specific heat of 1.1 J/gºC
and a mass of 385 g if it starts out at a temperature of 19.5°C and 885 J of
energy are added to it.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2


You know the right answer?
Please help I’m stuck
b. Water has a specific heat of 4.184 J/gºC. If the temperature of 250 g of<...
Questions






English, 21.01.2021 14:00














Mathematics, 21.01.2021 14:00