
Chemistry, 06.12.2021 03:40 loganparrish2488
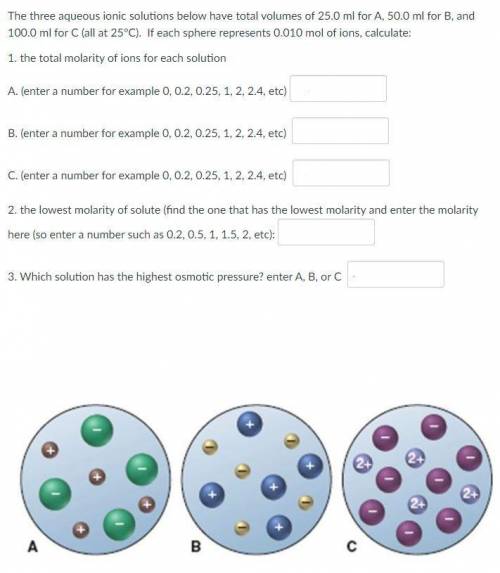
The three aqueous ionic solutions below have total volumes of 25.0 ml for A, 50.0 ml for B, and 100.0 ml for C (all at 25°C). If each sphere represents 0.010 mol of ions, calculate:
1. the total molarity of ions for each solution
2. the lowest molarity of solute
3. Which solution has the highest osmotic pressure?
See the picture attached.
My answers:
1.
A. 3.2
B. 2
C. 1.2
2. 0.4
3. A
Am I right?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 22.06.2019 21:20
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2
You know the right answer?
The three aqueous ionic solutions below have total volumes of 25.0 ml for A, 50.0 ml for B, and 100....
Questions

Business, 28.02.2020 19:00





History, 28.02.2020 19:00







History, 28.02.2020 19:00


Mathematics, 28.02.2020 19:01

Mathematics, 28.02.2020 19:01

English, 28.02.2020 19:01






