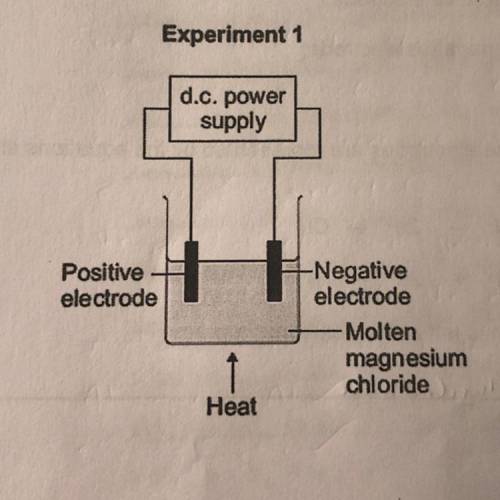
Explain how magnesium is produced at the negative electrode in experiment 1.
...

Chemistry, 15.11.2021 01:00 ibrsayed6671
Explain how magnesium is produced at the negative electrode in experiment 1.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
Questions

Business, 01.09.2019 17:10



Mathematics, 01.09.2019 17:10

Mathematics, 01.09.2019 17:10






Health, 01.09.2019 17:10



Mathematics, 01.09.2019 17:10

Geography, 01.09.2019 17:10

History, 01.09.2019 17:10


English, 01.09.2019 17:10

Physics, 01.09.2019 17:10

Mathematics, 01.09.2019 17:10



