
Chemistry, 26.10.2021 19:30 lazavionadams81
A 50g block of aluminum at -100°C is submerged in 30g of water at 20°C.
Cwater = 4.184 J/gºC
AH tus water = 334 J/g
Cu = 0.89 J/gºC
What is the final temperature of both substances?
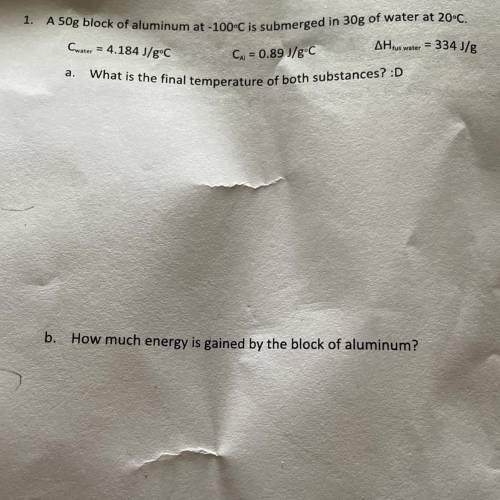

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 12:50
5. how can you decrease the pressure of a gas in a container without changing the volume of the gas?
Answers: 1

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
A 50g block of aluminum at -100°C is submerged in 30g of water at 20°C.
Cwater = 4.184 J/gºC
...
...
Questions


Advanced Placement (AP), 14.02.2020 01:14

Mathematics, 14.02.2020 01:14

Computers and Technology, 14.02.2020 01:14


English, 14.02.2020 01:14

Mathematics, 14.02.2020 01:14





Mathematics, 14.02.2020 01:14



Mathematics, 14.02.2020 01:14

Mathematics, 14.02.2020 01:14

Mathematics, 14.02.2020 01:15

Law, 14.02.2020 01:15

Mathematics, 14.02.2020 01:15



