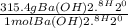WILL GIVE BRAINLIEST AND 20 POINTS! Barium hydroxide, often used to titrate weak organic acids, is obtained as the octahydrate, Ba(OH)2 * 8 H2O. What mass of Ba(OH)2 * 8 H2O would be required to make 500 mL of a solution that is 0.1500 M hydroxide ions? [hint: calculate the molar mass of barium hydroxide octahydrate].

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1


Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 2
You know the right answer?
WILL GIVE BRAINLIEST AND 20 POINTS!
Barium hydroxide, often used to titrate weak organic acids, is...
Questions

History, 10.09.2021 01:00



Mathematics, 10.09.2021 01:00

Mathematics, 10.09.2021 01:00


Mathematics, 10.09.2021 01:00

Spanish, 10.09.2021 01:00



English, 10.09.2021 01:00


Mathematics, 10.09.2021 01:00

Mathematics, 10.09.2021 01:00



Mathematics, 10.09.2021 01:00



Mathematics, 10.09.2021 01:00

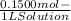 ×
×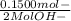 ×
×