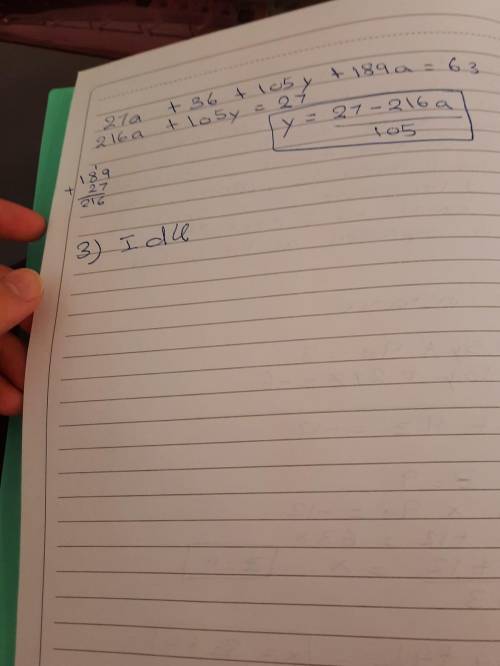1-Link the concepts of mole, molar mass, number of atoms, Avogadro’s
number and mass using a flowchart or mind map.
2-Rearrange the two equations to make each variable the focus (you will end up with three variations for each equation).
3- Record the equations for number of moles and number of particles (and
each equation triangle) - Annotate each symbol with what it means and definition and the units.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

You know the right answer?
1-Link the concepts of mole, molar mass, number of atoms, Avogadro’s
number and mass using a flowc...
Questions






Mathematics, 17.09.2019 06:30

Mathematics, 17.09.2019 06:30


English, 17.09.2019 06:30

English, 17.09.2019 06:30


History, 17.09.2019 06:30

Chemistry, 17.09.2019 06:30

Mathematics, 17.09.2019 06:30

Physics, 17.09.2019 06:30


History, 17.09.2019 06:30

Mathematics, 17.09.2019 06:30

Social Studies, 17.09.2019 06:30

English, 17.09.2019 06:30