
Chemistry, 11.10.2021 18:40 donnafranks2003
An excess of sodium carbonate, Na, CO3, in solution is added to a solution containing 17.87 g CaCl2. After performing the experiment, 13.19 g of calcium carbonate, CaCO3, is produced. Calculate the percent yield of this reaction.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the mass in grams of hydrogen gas produced when 14.0 moles of hcl is added to an excess amount of magnesium.
Answers: 3

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
You know the right answer?
An excess of sodium carbonate, Na, CO3, in solution is added to a solution containing 17.87 g CaCl2....
Questions

Mathematics, 23.02.2021 02:40

Mathematics, 23.02.2021 02:40



History, 23.02.2021 02:40

Mathematics, 23.02.2021 02:40

Mathematics, 23.02.2021 02:40




Mathematics, 23.02.2021 02:40



Mathematics, 23.02.2021 02:40

Mathematics, 23.02.2021 02:40

History, 23.02.2021 02:40

Biology, 23.02.2021 02:40

Social Studies, 23.02.2021 02:40


Mathematics, 23.02.2021 02:40

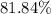 .
.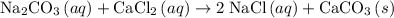 .
.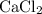 , as well as those in the product of interest,
, as well as those in the product of interest, 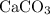 :
: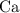 :
: 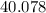 .
.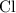 :
: 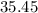 .
. :
: 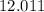 .
. :
:  .
.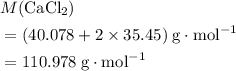 .
.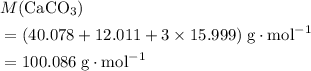 .
.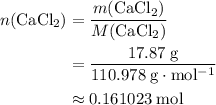 .
.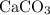 ) are both
) are both  . Thus:
. Thus: .
. 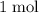 of
of 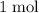 of
of 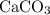 in this experiment:
in this experiment: .
. .
. , calculate the percentage yield of this experiment:
, calculate the percentage yield of this experiment: .
.

