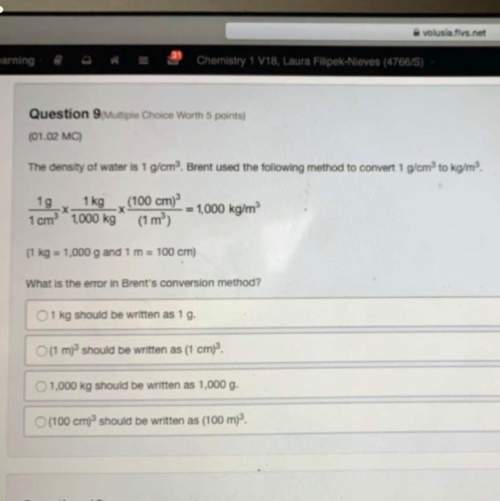
Calculate ΔH for the following reaction:
PCl3 (l) + Cl2 (g) → PCl5 (s)
given the follo...

Chemistry, 11.10.2021 14:00 tiaharris3191
Calculate ΔH for the following reaction:
PCl3 (l) + Cl2 (g) → PCl5 (s)
given the following:
1) 2 P (s) + 3 Cl2 (g) → 2 PCl3 (l) ΔH = -639.4 kJ
2) 2 P (s) + 5 Cl2 (g) → 2 PCl5 (s) ΔH = -887.0 kJ

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1
You know the right answer?
Questions




Mathematics, 26.08.2020 16:01








Mathematics, 26.08.2020 16:01


Mathematics, 26.08.2020 16:01




Business, 26.08.2020 16:01

Mathematics, 26.08.2020 16:01

Mathematics, 26.08.2020 16:01