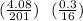Chemistry, 10.10.2021 21:40 skyemichellec
A compound of mercury and oxygen is heated in order to decompose the compound. A 4.08 grams sample of mercury oxide upon heating gives off oxygen gas. After heating the mass of mercury metal left behind was 3.78 grams. Calculate the empirical formula of the compound
My and: 1 O2 to 2 Hg ratio but I don't know if it's correct and if so if I should write it as
2HgO > 2Hg + O2 or just 2Hg + O2
Please someone who understands help.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3


Chemistry, 22.06.2019 23:30
The comparison of the number of atoms in a copper coin the size of a penny with the number of people on earth is made to illustrate which of the following? a. that atoms are indivisible b. that atoms are very small c. that atoms are very large d. that in a copper penny, there is one atom for every person on earth
Answers: 1
You know the right answer?
A compound of mercury and oxygen is heated in order to decompose the compound. A 4.08 grams sample o...
Questions




Mathematics, 16.10.2019 00:40



Physics, 16.10.2019 00:40


Mathematics, 16.10.2019 00:40


Geography, 16.10.2019 00:40

Physics, 16.10.2019 00:40



Biology, 16.10.2019 00:40

Geography, 16.10.2019 00:40

English, 16.10.2019 00:40

Mathematics, 16.10.2019 00:40

Mathematics, 16.10.2019 00:40