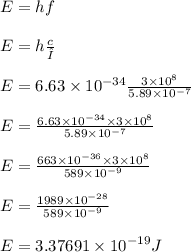Calculate the energy of one photon of yellow light that has a wavelength of 5.89x10^-7m.
...

Chemistry, 04.10.2021 08:40 Lilabsterdoll
Calculate the energy of one photon of yellow light that has a wavelength of 5.89x10^-7m.


Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2
You know the right answer?
Questions





Mathematics, 13.01.2021 01:00

Social Studies, 13.01.2021 01:00

Mathematics, 13.01.2021 01:00

Social Studies, 13.01.2021 01:00

Mathematics, 13.01.2021 01:00

Mathematics, 13.01.2021 01:00

Biology, 13.01.2021 01:00


English, 13.01.2021 01:00

Mathematics, 13.01.2021 01:00



Mathematics, 13.01.2021 01:00

Chemistry, 13.01.2021 01:00


Computers and Technology, 13.01.2021 01:00