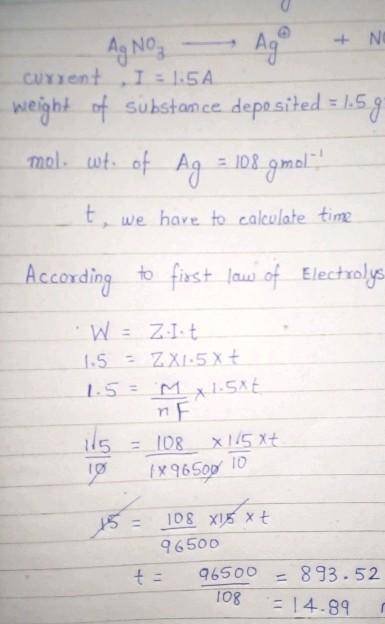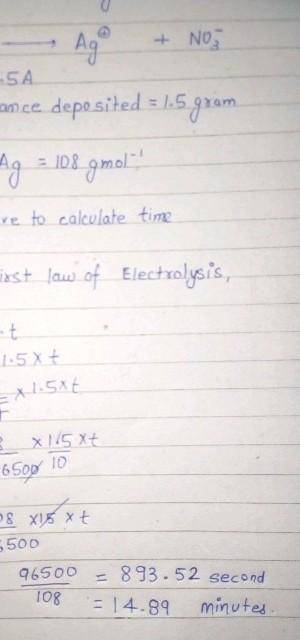Chemistry, 10.09.2021 17:20 rachelshryock551
calculate the amount of current that must flow for 50 minutes to a solution of silver trioxonitrate (V) solution to deposit 2 mole of silver. (Ag=108, Faraday's constant=96500C mol¯¹)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2
You know the right answer?
calculate the amount of current that must flow for 50 minutes to a solution of silver trioxonitrate...
Questions

Computers and Technology, 10.11.2020 16:30


Arts, 10.11.2020 16:30





Arts, 10.11.2020 16:30










Computers and Technology, 10.11.2020 16:30