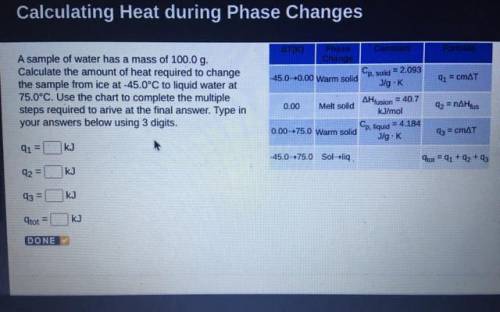
A sample of water has a mass of 100.0 g.
Calculate the amount of heat required to change
the...

A sample of water has a mass of 100.0 g.
Calculate the amount of heat required to change
the sample from ice at -45.0°C to liquid water at
75.0°C. Use the chart to complete the multiple
steps required to arive at the final answer. Type in
your answers below using 3 digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 23:00
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
Questions







Mathematics, 12.03.2020 00:33

Mathematics, 12.03.2020 00:33




Social Studies, 12.03.2020 00:33

English, 12.03.2020 00:33









