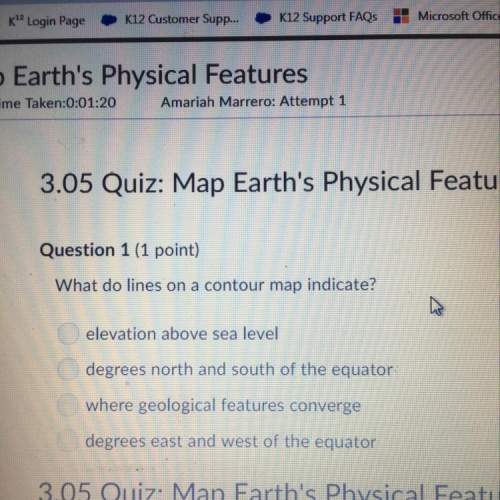
One kmol of CO and 0.5 kmol of O2 react to form a mixture at temperature T and pressure p consisting of CO2, CO, and O2. If 0.35 kmol of CO is present in an equilibrium mixture when the pressure is 1 atm, determine the amount of CO present in an equilibrium mixture at the same temperature if the pressure were 10 atm.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which one of the following gases is not an important component of soil?
Answers: 2

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2

Chemistry, 23.06.2019 01:00
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1

Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1
You know the right answer?
One kmol of CO and 0.5 kmol of O2 react to form a mixture at temperature T and pressure p consisting...
Questions

Physics, 12.10.2019 14:20


History, 12.10.2019 14:20


Social Studies, 12.10.2019 14:20

English, 12.10.2019 14:20

History, 12.10.2019 14:20

Mathematics, 12.10.2019 14:20

Chemistry, 12.10.2019 14:20




Mathematics, 12.10.2019 14:20

English, 12.10.2019 14:20





Chemistry, 12.10.2019 14:30