
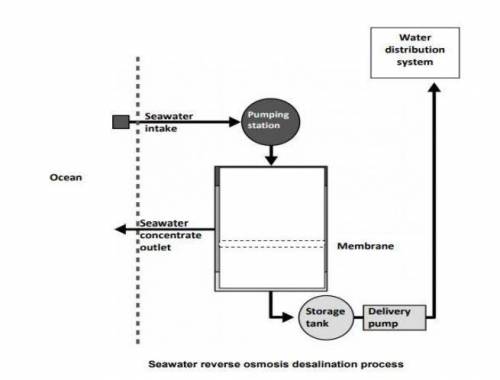
One way of producing drinking water from seawater is by reverse osmosis.
Reverse osmosis is a type of filtration. Seawater is pushed through a semi-permeable membrane. Pressure is applied to the seawater. Semi-permeable
means salt is trapped on one side of the membrane, but water can pass through.
The trapped salts form a ‘seawater concentrate’ on one side of the membrane.
Where do the trapped salts go?
[a] ocean [b]Water distribution system
[c]Storage tank [d]delivery pipeline
QUESTION 3:
Based on the above picture what will determine whether a particle is able to pass
through the membrane?
[a] particle size [b] particle mass
[c] number of particles [d] charge on the particle

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2

Chemistry, 23.06.2019 07:00
Why do the strengths of london (dispersion) forces generally increase with increasing molecular size? choose one: a. heavier atoms have stronger attractions for each other than lighter atoms. b. dispersion forces are all equal in magnitude; there is no size dependence. c. dispersion forces arise from the attraction between the nuclei of atoms, and larger molecules have larger nuclei. d. dispersion forces arise from dipoles caused by the electron distribution being distorted. larger molecules have more electrons and, therefore, more distortions and a bigger force. e. dispersion forces depend on distance. larger molecules are farther apart and so the forces are smaller.
Answers: 2
You know the right answer?
One way of producing drinking water from seawater is by reverse osmosis.
Reverse osmosis is a type...
Questions

Computers and Technology, 25.07.2019 20:00

Mathematics, 25.07.2019 20:00


Mathematics, 25.07.2019 20:00



English, 25.07.2019 20:00

Mathematics, 25.07.2019 20:00

Health, 25.07.2019 20:00

History, 25.07.2019 20:00

Mathematics, 25.07.2019 20:00

Mathematics, 25.07.2019 20:00

English, 25.07.2019 20:00

Chemistry, 25.07.2019 20:00

Biology, 25.07.2019 20:00

Chemistry, 25.07.2019 20:00


Mathematics, 25.07.2019 20:10


History, 25.07.2019 20:10



