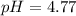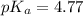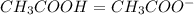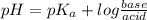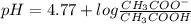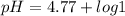Calculating the expected pH of the buffer solution: Given that the pKa for Acetic Acid is 4.77, calculate the expected pH of the buffer solutions using the Henderson-Hasselbalch equation and the concentrations of Acetic Acid and Acetate added to the 250 ml Erlenmeyer flask: pH

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 3

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2
You know the right answer?
Calculating the expected pH of the buffer solution: Given that the pKa for Acetic Acid is 4.77, calc...
Questions



Chemistry, 12.04.2021 23:50


Arts, 12.04.2021 23:50

History, 12.04.2021 23:50

Mathematics, 12.04.2021 23:50

Health, 12.04.2021 23:50


Computers and Technology, 12.04.2021 23:50





Advanced Placement (AP), 12.04.2021 23:50

Mathematics, 12.04.2021 23:50

Mathematics, 12.04.2021 23:50



Mathematics, 12.04.2021 23:50