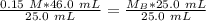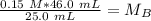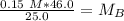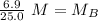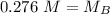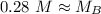Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1
You know the right answer?
A titration is performed on a 25.0 mL sample of calcium hydroxide. A volume of
46.0 mL of a 0.15 M...
Questions



Mathematics, 21.07.2020 16:01

Mathematics, 21.07.2020 16:01

Mathematics, 21.07.2020 16:01

Mathematics, 21.07.2020 16:01

Social Studies, 21.07.2020 16:01



Computers and Technology, 21.07.2020 16:01




Mathematics, 21.07.2020 16:01


Mathematics, 21.07.2020 16:01


Computers and Technology, 21.07.2020 16:01



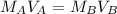

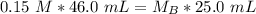
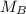 . It is being multiplied by 25.0 milliliters. The inverse operation of multiplication is division, so we divide both sides of the equation by 25.0 mL.
. It is being multiplied by 25.0 milliliters. The inverse operation of multiplication is division, so we divide both sides of the equation by 25.0 mL.