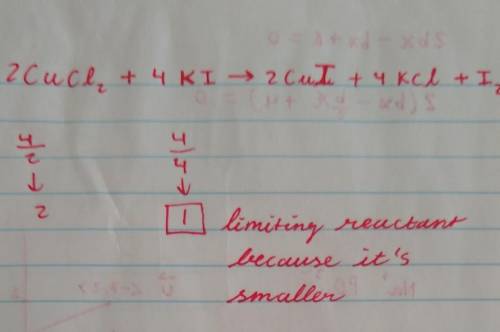Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2
You know the right answer?
Consider the reaction 2CuCl2 + 4KI → 2CuI + 4KCl + I2. If 4 moles of CuCl2 react with 4 moles of KI,...
Questions

Social Studies, 17.09.2019 16:50

History, 17.09.2019 16:50


Health, 17.09.2019 16:50


Social Studies, 17.09.2019 16:50


Computers and Technology, 17.09.2019 16:50


History, 17.09.2019 16:50

History, 17.09.2019 16:50

Mathematics, 17.09.2019 16:50



Business, 17.09.2019 16:50


Social Studies, 17.09.2019 16:50



Mathematics, 17.09.2019 16:50