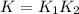Chemistry, 14.07.2021 03:40 brodytheforce
Hydrogen is manufactured on an industrial scale by this sequence of reactions: Write an equation that gives the overall equilibrium constant in terms of the equilibrium constants and . If you need to include any physical constants, be sure you use their standard symbols, which you'll find in the ALEKS Calculator.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3


Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
You know the right answer?
Hydrogen is manufactured on an industrial scale by this sequence of reactions: Write an equation tha...
Questions

Chemistry, 30.06.2019 19:00




Mathematics, 30.06.2019 19:00



Social Studies, 30.06.2019 19:00









Social Studies, 30.06.2019 19:00



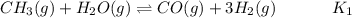
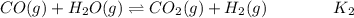
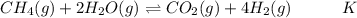
 in terms of the equilibrium constants
in terms of the equilibrium constants 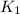 and
and 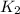 . If you need to include any physical constants, be sure you use their standard symbols, which you'll find in the ALEKS Calculator.
. If you need to include any physical constants, be sure you use their standard symbols, which you'll find in the ALEKS Calculator.![$K_1 = \frac{[CO][H_2]^3}{[CH_4][H_2O]}$](/tpl/images/1393/7713/f333b.png) ...............(1)
...............(1)![$K_2 = \frac{[CO_2][H_2]}{[CO][H_2O]}$](/tpl/images/1393/7713/f1f67.png) ...................(2)
...................(2)![$K=\frac{[CO_2][H_2]^4}{[CH_4][H_2O]^2}$](/tpl/images/1393/7713/c60b6.png)
![$K_1 \times K_2=\frac{[CO][H_2]^3}{[CH_4][H_2O]} \times \frac{[CO_2][H_2]}{[CO][H_2O]}$](/tpl/images/1393/7713/392a2.png)
![$K_1K_2=\frac{[CO_2][H_2]^4}{[CH_4][H_2O]^2}$](/tpl/images/1393/7713/90e50.png) .................(4)
.................(4)