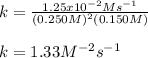NO2 (g) reacts with O2 4 NO2 (g) + O2(g) to produce N2 O5 (g) as shown by the equation below: (g) = 2 N2 O5 (g).
Experimentally the rate orders were determined and rate law written as shown below: Rate = k [NO2]2[O2 ].
Calculate the value of k if the initial concentration of NO2 was 0.250 M and initial
concentration of O2 (g) was 0.150 M. The initial rate was 1.25 x 10-2 M. s-1 .

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2

Chemistry, 22.06.2019 07:20
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1
You know the right answer?
NO2 (g) reacts with O2 4 NO2 (g) + O2(g) to produce N2 O5 (g) as shown by the equation below: (g) =...
Questions





Business, 19.09.2019 04:00








Mathematics, 19.09.2019 04:00



Health, 19.09.2019 04:00

Mathematics, 19.09.2019 04:00


History, 19.09.2019 04:00

Chemistry, 19.09.2019 04:00

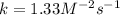
![k=\frac{r}{[NO_2]^2[O_2]}](/tpl/images/1392/3789/849e7.png)