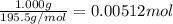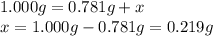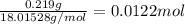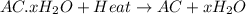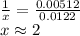A sample of unknown hydrate, AC-XH20, has a mass of 1.000 g before heating and a
mass of 0.781 g after heating. If the molar mass of the anhydrous compound (AC) is
195.5 g/mol, what is the water of crystallization for the formula of the unknown
hydrate?
Type your work for partial credit.
Answer choices: 2, 3, 5, or 6.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
A sample of unknown hydrate, AC-XH20, has a mass of 1.000 g before heating and a
mass of 0.781 g af...
Questions




Spanish, 24.04.2020 23:04






Mathematics, 24.04.2020 23:04






Mathematics, 24.04.2020 23:04



Chemistry, 24.04.2020 23:05

Social Studies, 24.04.2020 23:05