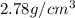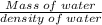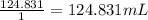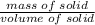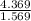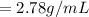A 4.369 g sample of metal is placed in a flask. Water is added to the flask and the total volume in the flask is read to be 126.4 ml. The mass of the water, flask, and metal is 268.5 g. If the mass of the flask is 139.3 g and the density of water is 1.000 g/mL, the density of the solid is g/cm3.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 02:30
24 points and brainliest to anyone who can answer under 10 minutes with best ! the table below shows the role of different substances during photosynthesis. substance role during photosynthesis glucose stores chemical energy water combines with glucose to form carbon dioxide chlorophyll traps sunlight which of the following statements would correct one of the roles listed in the table? glucose combines with carbon to form water. chlorophyll reacts with light to produce carbon dioxide. water combines with carbon dioxide during photosynthesis. chlorophyll stores chemical energy needed for photosynthesis.
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

You know the right answer?
A 4.369 g sample of metal is placed in a flask. Water is added to the flask and the total volume in...
Questions






English, 12.07.2019 18:30

Mathematics, 12.07.2019 18:30

Mathematics, 12.07.2019 18:30

Arts, 12.07.2019 18:30

Mathematics, 12.07.2019 18:30

Mathematics, 12.07.2019 18:30



English, 12.07.2019 18:30

Mathematics, 12.07.2019 18:30


History, 12.07.2019 18:30

Mathematics, 12.07.2019 18:30