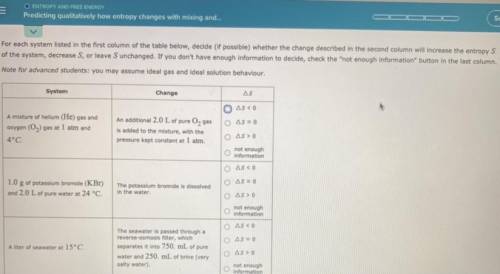
Please help! Thanks in advance.
...

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
The skeletal system performs a variety of functions that are crucial to maintaining life processes. what function is performed in the bone marrow, but not in the ossified bones of the skeleton? a oxygen transportation c mineral storage b. muscle attachment d red blood cell production
Answers: 3

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2

Chemistry, 23.06.2019 15:00
Does the formation of all chemical bonds is based on sharing of electrons?
Answers: 1
You know the right answer?
Questions


Engineering, 26.02.2021 05:50



Mathematics, 26.02.2021 05:50


Mathematics, 26.02.2021 05:50

English, 26.02.2021 05:50

Mathematics, 26.02.2021 05:50

Mathematics, 26.02.2021 05:50


Mathematics, 26.02.2021 05:50

Mathematics, 26.02.2021 05:50


Mathematics, 26.02.2021 05:50



Mathematics, 26.02.2021 05:50

Mathematics, 26.02.2021 05:50