
Chemistry, 24.06.2021 22:00 aryannaholmes9
Calculate the energy for the transition of an electron from the n=4 level to the n=6 level of a hydrogen atom.
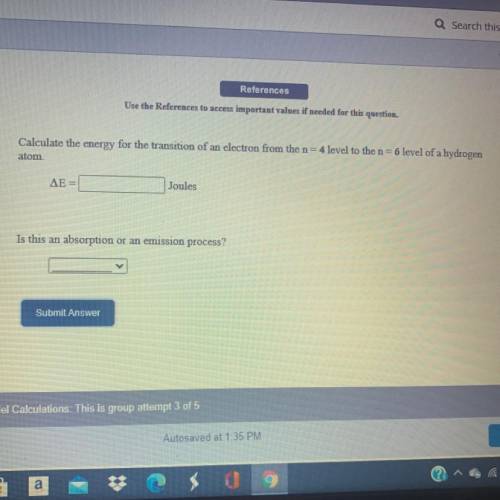

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
You know the right answer?
Calculate the energy for the transition of an electron from the n=4 level to the n=6 level of a hydr...
Questions

English, 12.09.2019 03:30



Health, 12.09.2019 03:30



Mathematics, 12.09.2019 03:30


Mathematics, 12.09.2019 03:30



Mathematics, 12.09.2019 03:30



Mathematics, 12.09.2019 03:30

Mathematics, 12.09.2019 03:30



Mathematics, 12.09.2019 03:30



