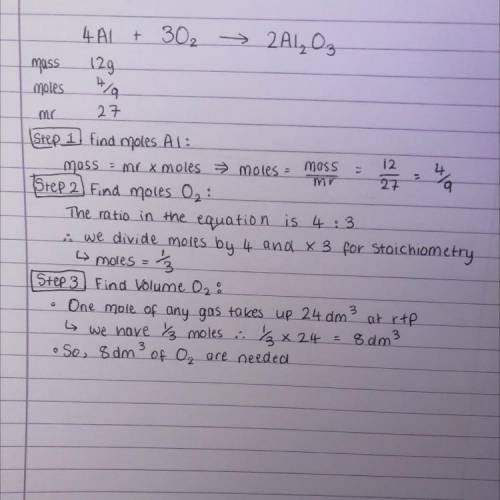Chemistry, 24.06.2021 20:50 lollipop8011
4. Aluminium combines with oxygen to form aluminium oxide via the equation below. When 12g of aluminium is reacted, calculate the volume of O2 that is needed at rtp. 4Al + 3O2 > 2Al2O3

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which mathematical relationship should you us to convert moles of a substance into grams
Answers: 1

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
4. Aluminium combines with oxygen to form aluminium oxide via the equation below. When 12g of alumin...
Questions



Chemistry, 20.02.2020 17:47

Social Studies, 20.02.2020 17:47



English, 20.02.2020 17:47







History, 20.02.2020 17:47

Mathematics, 20.02.2020 17:47


Mathematics, 20.02.2020 17:47