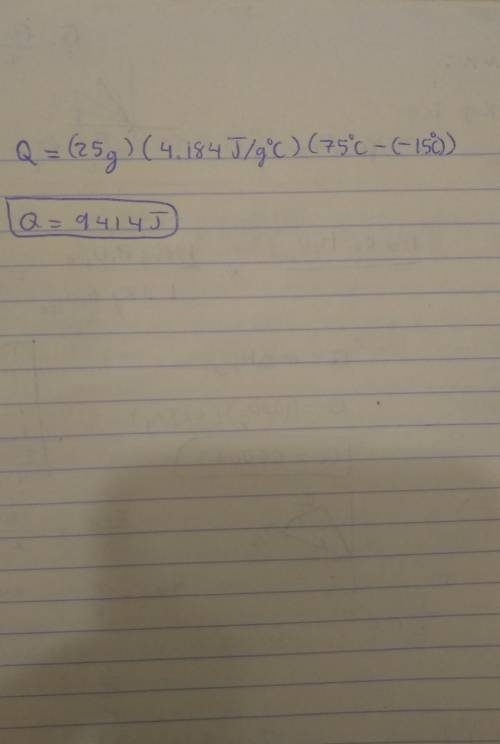Chemistry, 24.06.2021 03:30 sevaramirabell
Note: Please show all work and calculation setups to get full credit. T. he following may be used on this assignment: specific heat of (water=4.184 J/g oC; ice=2.03 J/g oC; steam=1.99 184 J/g oC); heat of fusion of water=80. cal/g; heat of vaporization=540 cal/g; 1cal=4.184J. Calculate the energy required (in J) to convert 25 g of ice at -15 oC to water at 75 oC.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Which of the following statements concerning the influence of culture on ethnic identity formation is accurate? a. one will reject ethnic identity if cultural stereotypes are encountered. b. if one’s ethnic city is different from the dominant cultural group, then one’s ethnic identity you will become weekend. c. if an the ethnic group is excepted by dominant culture, then ethnic identity formation can be a difficult process. d. similarity to the dominant culture can determine how easy it is for one to except ethnic differences.
Answers: 2

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1

You know the right answer?
Note: Please show all work and calculation setups to get full credit. T. he following may be used on...
Questions

Computers and Technology, 27.07.2019 23:00



History, 27.07.2019 23:00

Social Studies, 27.07.2019 23:00






Biology, 27.07.2019 23:00




Social Studies, 27.07.2019 23:00


History, 27.07.2019 23:00



History, 27.07.2019 23:00