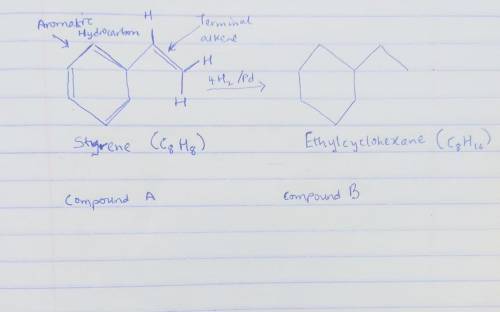
Compound A has the formula C8H8. It reacts rapidly with acidic KMnO4 but reacts with only 1 equivalent of H2 over a palladium catalyst. On hydrogenation under conditions that reduce aromatic rings, A reacts with 4 equivalents of H2, and hydrocarbon B, C8H16, is produced. The reaction of A with KMnO4 gives CO2 and a carboxylic acid C, C7H6O2.
Required:
Draw the structure of compound B below.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1



Chemistry, 23.06.2019 05:30
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3
You know the right answer?
Compound A has the formula C8H8. It reacts rapidly with acidic KMnO4 but reacts with only 1 equivale...
Questions

Mathematics, 19.05.2021 17:30

Biology, 19.05.2021 17:30

History, 19.05.2021 17:30


Health, 19.05.2021 17:30


Mathematics, 19.05.2021 17:30


Mathematics, 19.05.2021 17:30




Physics, 19.05.2021 17:30

Mathematics, 19.05.2021 17:30

Mathematics, 19.05.2021 17:30


History, 19.05.2021 17:30

Mathematics, 19.05.2021 17:30


Mathematics, 19.05.2021 17:30