Chemistry, 21.06.2021 20:50 kaylatunell123
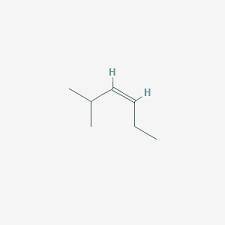
Compounds X and Y both have the formula C7H14. Both X and Y react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form 2-methylhexane. The heat of hydrogenation of X is greater than that of Y. Both X and Y react with HCl to give the same single C7H15Cl compound as the major product. What is the structure of X?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 23.06.2019 07:00
Agas has an empirical formula ch4. 0.16g of the gas occupies a volume of 240cm^3 what is the molecular formula of the me anyone who !
Answers: 1
You know the right answer?
Compounds X and Y both have the formula C7H14. Both X and Y react with one molar equivalent of hydro...
Questions


Advanced Placement (AP), 21.01.2020 21:31

Mathematics, 21.01.2020 21:31

Mathematics, 21.01.2020 21:31

Social Studies, 21.01.2020 21:31

Physics, 21.01.2020 21:31


History, 21.01.2020 21:31

Mathematics, 21.01.2020 21:31



Mathematics, 21.01.2020 21:31

Mathematics, 21.01.2020 21:31

Mathematics, 21.01.2020 21:31

Mathematics, 21.01.2020 21:31





Biology, 21.01.2020 21:31